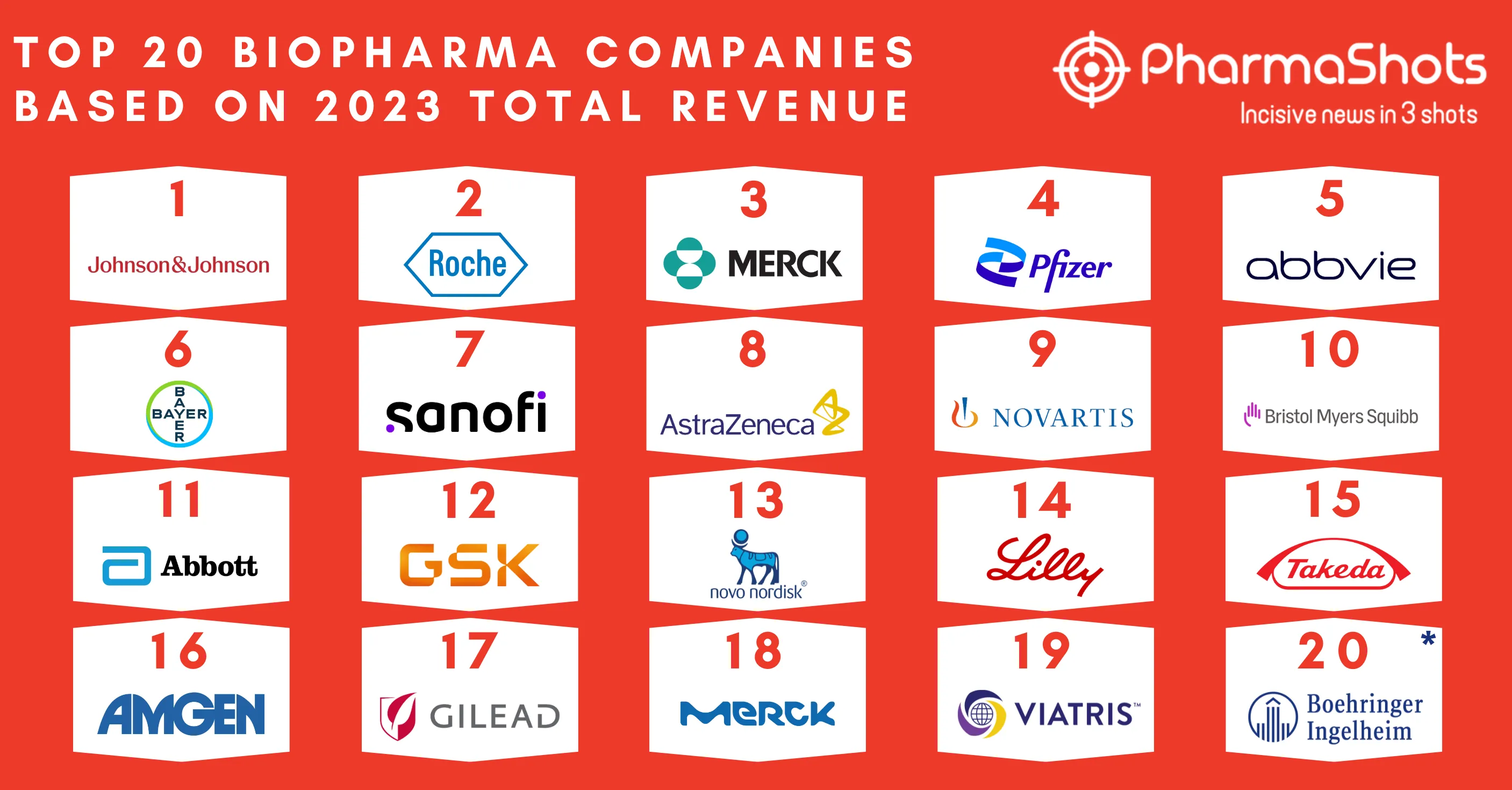
Top 20 Vaccines Based on 2022 Total Revenue
Shots:
- Driven by a noble pursuit to ensure global health security, vaccines cater to the healthcare needs of millions and billions of people. With an instrumental role in containing the COVID-19 pandemic, immunization prevents more than 5 million deaths annually from conditions like diphtheria, tetanus, pertussis, influenza, and measles
- With a global market size of $41.4B in 2021, it is anticipated to reach $67.2B by 2026. Pfizer and BioNTech's Comirnaty secured the apical position by generating $37.81B, followed by Merck & Co.'s Gardasil/Gardasil-9 and Pfizer's Prevnar family in the second and third position respectively in 2022
- PharmaShots brings an informative report on the Top 20 Vaccines of the year 2022 based on the total revenue generated

Company: Merck & Co.
First Approved: US (Mar 29, 1996), EU (1991)
Total Revenue: $173M
Indications Approved: Hepatitis A Vaccine
Vaqta is an intramuscular injection administered to prevent disease caused by the Hepatitis A Virus (HAV) in pediatrics aged 12 months or older. The vaccine triggers the immune activation of lymphocytes to engulf viral antigens, releasing inflammatory mediators that stimulate B and T cells to attack viral antigens. The stimulation of B and T cells converts them into memory cells, antibody-producing B cells, cytotoxic T cells, and helper T cells to provide immunity against infection with hepatitis A. Vaqta’s 2022 revenue decreased by 3.35% in comparison to 2021.

Company: AstraZeneca
First Approved: US (Jun 18, 2003), EU (Dec 04, 2013)
Total Revenue: $175M
Indications Approved: Active immunization to prevent tick-borne encephalitis disease
FluMist is prescribed for the active immunization and prevention of influenza disease caused by influenza A subtype viruses and type B viruses contained in the vaccine. It is a live attenuated vaccine that provides immunization against viral entry and infections. The vaccine stimulates the production of mucosal IgA, systemic IgG, and T cells. FluMist’s revenue in the year 2022 faced a recession of 30.83% as compared to the year 2021.

Company: Pfizer
First Approved: US (Aug 13, 2021), EU (N/A)
Total Revenue: $200M
Indications Approved: Active immunization to prevent tick-borne encephalitis disease
Ticovac is an inactivated viral vaccine developed to act against meningoencephalitis caused by the Tick-borne Encephalitis (TBE) virus. It contains an inactivated whole virus, either Neudoerfl or Karlsruhe (K23) strain of the TBEV-Eu subtype. This vaccine induces neutralization of antibodies against the natural TBE virus and thereby allowing protection from Tick-borne Encephalitis (TBE). Ticovac’s 2022 revenue rose by 8.11% vs 2021.

Company: GSK
First Approved: US (Jun 03, 2022), EU (May 25, 2012)
Total Revenue: $227.51M
Indications Approved: Measles Prophylaxis, Mumps Prophylaxis, Rubella Prophylaxis
Priorix is indicated for active immunization and the prevention of Measles, Mumps, and Rubella (MMR) in pediatrics aged 12 months or older. It is a lyophilized mixed preparation of the attenuated Schwarz measles, RIT 4385 mumps, and Wistar RA 27/3 rubella strains of viruses. By producing neutralizing antibodies, the vaccine protects against MMR by developing humoral immune responses against the MMR viruses. Priorix’s 2022 revenue declined by 35.35% as compared to 2021.

Company: Pfizer
First Approved: US (Dec 28, 2022), EU (Apr 20, 2012)
Total Revenue: $268M
Indications Approved: Active immunization against invasive meningococcal ACWY disease
Nimenrix provides an active immunization against invasive meningococcal diseases caused by Neisseria meningitidis groups A, C, W-135, and Y in individuals aged six weeks or more. This anti-capsular meningococcal antibody protects the human body against meningococcal disease through complement-mediated bactericidal. The vaccine induces the production of bactericidal antibodies against capsular polysaccharides of Neisseria meningitidis groups A, C, W-135, and Y. Nimenrix’s revenue in 2022 inclined by 38.86% vs 2021.

Company: GSK
First Approved: US (N/A), EU (Mar 30, 2009)
Total Revenue: $369.09M
Indications Approved: Invasive Disease, Pneumonia, Acute Otitis Media
Synflorix is a pneumococcal polysaccharide conjugate vaccine developed for the treatment of invasive diseases, pneumonia, and acute otitis media in infants and children aged between 6 weeks to 5 years. It is composed of 10 active ingredients including the S. pneumoniae polysaccharide serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F, each conjugated to a carrier protein (mainly protein D, tetanus toxoid, or diphtheria toxoid). The carrier protein provides T-cells help to B-cells to produce an antibody response of high affinity to the polysaccharide antigen. Synflorix’s 2022 revenue decreased by 23.62% vs 2021.

Company: GSK
First Approved: US (Feb 19, 2010), EU (Mar 15, 2010)
Total Revenue: $417.50M
Indications Approved: Meningitis
Menveo is indicated for active immunization against invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135 in individuals aged 2 months up to 55 years. The vaccine contains small amounts of oligosaccharides extracted from the four serogroups A, C, Y, and W-135 that are conjugated to a protein from the bacterium Corynebacterium diphtheria. It functions by protecting against invasive meningococcal disease through the presence of serum bactericidal antibodies. Menveo’s revenue boosted by 13.39% in 2022 vs 2021.
Company: Merck & Co.
First Approved: US (Jun 01, 1983), EU (N/A)
Total Revenue: $602M
Indications Approved: Provides immunization for the prevention of pneumococcal disease
Pneumovax 23 is prescribed for the treatment of serious infections like ear infections, sinus infections, pneumonia, blood infection, and meningitis caused by the bacteria Streptococcus pneumoniae. It is a concoction of 23 serotypes extracted from Streptococcus pneumoniae bacteria, including 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F. Upon administering the pneumococcal vaccine, the body recognizes these serotypes as foreign entities and produces antibodies to act against them. Pneumovax 23’s 2022 revenue decreased by 32.59% as compared to 2021.

Company: GSK
First Approved: US (Apr 03, 2008), EU (Feb 21, 2006)
Total Revenue: $637.75M
Indications Approved: Rotavirus
Rotarix prevents rotavirus gastroenteritis caused by G1 and non-G1 types, including G3, G4, and G9. It is a live attenuated rotavirus vaccine derived from the human 89-12 strain belonging to G1P[8] type. As the exact mechanism of action by which Rotarix functions is unknown, it is believed that the vaccine induces immunity by replicating in the small intestine. Rotarix’s revenue decreased by 12.91% in 2022 as compared to 2021.

Company: GSK
First Approved: US (May 03, 2005), EU (Sep 24, 2010)
Total Revenue: $718.83M
Indications Approved: Diphtheria, Tetanus, Acellular Pertussis Booster
Boostrix is a non-infectious sterile vaccine prescribed for the treatment of active booster immunization against tetanus, diphtheria, and pertussis in individuals aged ten years or above. It may also be indicated during the third trimester of pregnancy to prevent pertussis in infants younger than two months of age. The vaccine functions by inducing an antibody response against all the vaccine components. Boostrix’s revenue in 2022 increased by 1.93% vs 2021.

Company: GSK
First Approved: US (Jul 08, 2003), EU (Oct 23, 2000)
Total Revenue: $718.83M
Indications Approved: Diphtheria, Tetanus, Pertussis, Polio, Hepatitis B, Haemophilus Influenza Type B
Infanrix is indicated for the active immunization against diphtheria, tetanus, and pertussis in infants and children 6 weeks to 6 years of age as a 5-dose series. In contrast, Pediarix is indicated for the active immunization against diphtheria, tetanus, pertussis, and infections caused by all known subtypes of the hepatitis B virus. Both Infanrix and Pediarix provide immunity against these indications by inducing the production of antibodies and by mounting an immunological memory. The revenue for Infanrix and Pediarix decreased by 2.19% in 2022 as compared to 2021.

Company: Merck & Co.
First Approved: US (Feb 03, 2006), EU (Jun 27, 2006)
Total Revenue: $783M
Indications Approved: Rotavirus Vaccine
RotaTeq is a vaccine that provides prevention from the rotavirus gastroenteritis caused by types G1, G2, G3, G4, and G9 in infants aged between 6 to 32 weeks. It contains a live attenuated virus that gets replicated in the intestine and interacts with the patient’s immune system to produce immunity. The exact mechanism by which the rotavirus vaccine interacts with the immune system is not specified. RotaTeq’s 2022 revenue declined by 2.97% as compared to the year 2021.

Company: GSK
First Approved: US (Dec 14, 2012), EU (Dec 12, 2018)
Total Revenue: $864.05M
Indications Approved: Influenza
Fluarix is indicated for the active immunization of children aged group 6 months to adults up to 64 years of age against influenza disease caused by influenza A and B virus subtypes. Administered in the form of intramuscular injection, Fluarix is prepared from an inactivated form of the influenza vaccine containing antigens propagated in embryonated eggs. The vaccine functions by developing immunity against the influenza virus by stimulating the production of antibodies specific to the disease. Fluarix’s revenue in 2022 decreased by 5.99% vs 2021.

Company: GSK
First Approved: US (Jan 23, 2015), EU (Jan 14, 2013)
Total Revenue: $911.25M
Indications Approved: Meningitis
Bexsero is a multicomponent meningococcal serogroup B vaccine indicated for active immunization from invasive disease caused by Neisseria meningitidis serogroup B. The vaccine allows the production of antibodies directed against NHBA, NadA, fHbp, and PorA P1.4, the proteins found on the surface of meningococci. It allows protection against invasive meningococcal disease mainly through complement-mediated antibody-dependent killing of N. meningitidis. Bexsero’s 2022 revenue inclined by 3.57% as compared to 2021.

Company: AstraZeneca
First Approved: US (N/A), EU (Oct 31, 2022)
Total Revenue: $1.79B
Indications Approved: COVID-19
Vaxzevria is an adenovirus vector vaccine indicated for the prevention of COVID-19 caused by SARS-CoV-2 in individuals aged 18 years and above. This vaccine is a replication-deficient chimpanzee adenovirus vector that encodes a trimeric pre-fusion form of the SARS-CoV-2 spike protein. Following its infusion, the spike proteins are expressed locally thereby allowing the immune system to generate a neutralizing antibody or cellular immune response. Vaxzevria’s revenue declined by 26.29% in 2022 as compared to 2021.

Company: Johnson & Johnson
First Approved: US (N/A), EU (Jan 09, 2023)
Total Revenue: $2.18B
Indications Approved: COVID-19
Jcovden is a vaccine indicated for the prevention of COVID-19 caused by SARS-CoV-2 in patients aged 18 years and above. It is a monovalent vaccine developed using a recombinant, replication-incompetent human adenovirus type 26 vector that encodes SARS-CoV-2 full-length spike glycoprotein. Following its administration, the S glycoprotein of SARS-CoV-2 is transiently expressed, inducing immunological responses against the S antigen as well as neutralizing and other functional S-specific antibodies that help protect against COVID-19. Jcovden’s revenue in the year 2022 dropped by 8.64% vs 2021.

Company: Merck & Co.
First Approved: US (Mar 17, 1995), EU (Apr 21, 2021)
Total Revenue: $2.24B
Indications Approved: Active Immunization for the Prevention of Varicella (Chickenpox)
Varivax develops active immunization against chickenpox in individuals aged 12 months and above. The vaccine contains the Oka/Merck strain of live, attenuated varicella virus, which was initially obtained from a child with wild-type varicella. The vaccine protects against chickenpox by generating both cell-mediated and humoral immune responses against the varicella-zoster virus. Varivax’s 2022 revenue increased by 4.96% as compared to 2021.

Company: Pfizer
First Approved: US (Prevnar 13- Feb 24, 2010, Prevnar 20 - Jun 08, 2021), EU (Prevnar 13 - Feb 14, 2022, Prevnar 20 - Dec 09, 2009)
Total Revenue: $6.34B
Indications Approved: Pneumococcal Disease Prophylaxis
The Prevnar Family comprises Prevnar 13 and Prevnar 20. Prevnar 13 is indicated for active immunization from invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A 19F, and 23F in individuals aged between 6 weeks to 5 years and for the prevention of otitis media caused by serotypes 4, 6B, 9V, 14, 18C, 19F and 23F. On the other hand, Prevnar 20 is indicated for the prevention of invasive disease caused by serotypes s 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F in individuals 6 weeks of age and older. Both Prevnar 13 and Prevnar 20 function by developing a T-cell-dependent immune response aided by polysaccharides conjugated to a carrier protein. The total revenue generated from the Prevnar Family in 2022 was boosted by 20.20% vs 2021.

Company: Merck & Co.
First Approved: - US (Gardasil - Jun 06, 2006, Gardasil-9 - Dec 10, 2014), EU (Gardasil - Oct 10, 2022, Gardasil-9 - Jun 10, 2015)
Total Revenue: $6.89B
Indications Approved: Gardasil - Human Papillomavirus Vaccines and Gardasil-9 – Human Papillomavirus 9-Valent
Gardasil is indicated for the treatment of Human Papillomavirus (HPV) among individuals aged 9 to 26 years. It is a non-infectious recombinant quadrivalent vaccine developed through purified (VLPs) of the major capsid protein of HPV types 6, 11, 16, and 18. Whereas, Gardasil-9 is indicated for the treatment of HPV among individuals aged 9 to 45 years. It is developed from the purified VLPs of the major capsid proteins of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Both vaccine function by developing a humoral immune response. The total revenue generated by both Gardasil and Gardasil-9 inclined by 21.57% in 2022 vs 2021.

Company: Pfizer and BioNTech
First Approved: US (Aug 23, 2021), EU (Oct 10, 2022)
Total Revenue: $37.81B
Indications Approved: COVID-19
Comirnaty is a vaccine prescribed for active immunization against COVID-19 caused by SARS-CoV-2 in individuals aged 12 years and older. The vaccine functions as the nucleoside-modified mRNA in it is formulated in lipid particles that enable the delivery of the mRNA into the host cells to allow the expression of the SARS-CoV-2 S antigen. It extracts both neutralizing antibodies and cellular immune responses to the S protein that helps protect against subsequent SARS-CoV-2 infection. Comirnaty’s revenue in the year 2022 rose by 2.79% as compared to the year 2021.
Sources:
- Annual reports
- SEC Filings
- Press releases
- Product websites
Currency Conversion: X-Rates (Jul 25, 2022)
Note:
- Vaccines mentioned in this report are from public companies
- All revenues are reported in $B
- The revenue for Fluarix Increased in GBP but Decreased in USD (Due to Currency Rate Drop)
- The revenue for Infanrix/Pediarix Increased in GBP but Decreased in USD (Due to Currency Rate Drop)
- The report only mentions the EU approval year of the vaccine Vaqta
Related Posts: Top 20 Nutraceutical Companies Based on 2022 Segment Revenue
Tags

Shivani is a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.